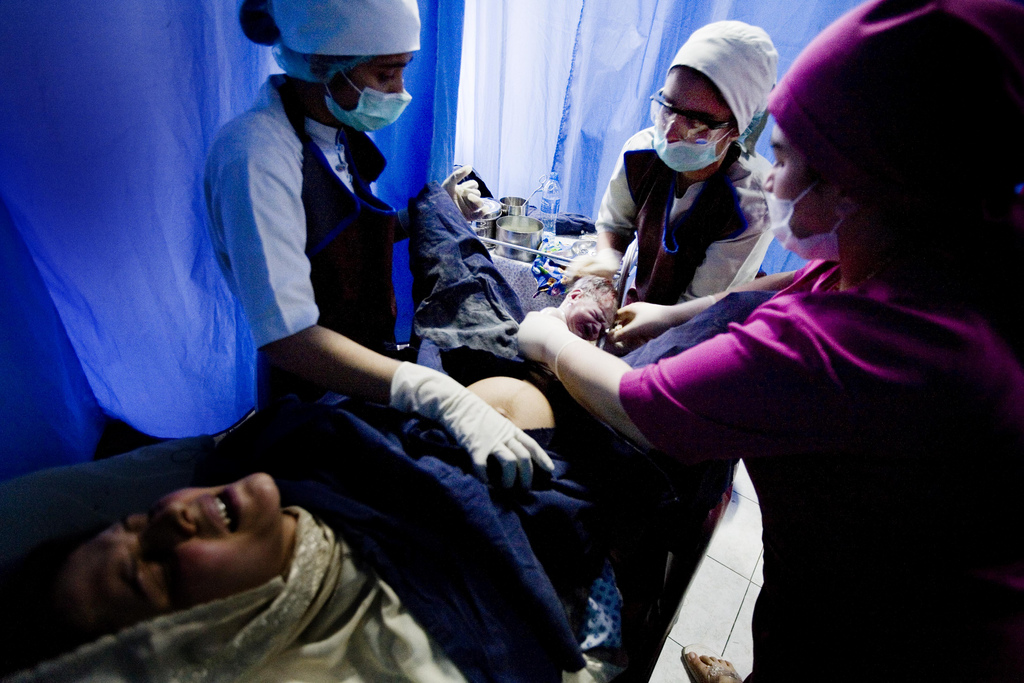
A young woman arrived at a health clinic in Sierra Leone with heavy bleeding. She was suffering from postpartum hemorrhage (PPH)—or excessive bleeding after birth—the most common cause of death for women after delivery.
The midwife at the clinic acted quickly, administering oxytocin, a uterotonic that helps the uterus contract to stop the bleeding. However, the facility was lacking the refrigeration needed to properly store the drug, which was also two years out of date. As a result, the oxytocin had no effect, and the woman died two hours later.
Tragically, poor and marginalized populations suffering from a disproportionate burden of disease often have the least access to high-quality health services. This is especially true of women during childbirth, who often deliver at home instead of health facilities. Those women who do make it to a facility may find them ill-equipped, lacking skilled personnel and essential medicines. Or, as in the case of the young woman from Sierra Leone, the medicines may be expired and improperly stored, thereby greatly diminishing their effectiveness.

Alarmingly, all of these women will be at risk of dying from PPH without access to uterotonics. And while oxytocin is the gold standard for preventing and treating PPH, it is not always available or kept sufficiently cool. It must also be given through injection by a skilled birth attendant, such as a doctor or nurse.
Thankfully, there is a second-line uterotonic drug that can be used to prevent and treat PPH when oxytocin is not available: misoprostol. The World Health Organization (WHO) recently added misoprostol — which does not require refrigeration and can be taken as a pill — to the Essential Medicines List for treatment of PPH in every country. This action expands the range of options to treat PPH, empowering health care workers with one more tool in their arsenal to fight bleeding after birth.
Here at USAID’s flagship Maternal and Child Survival Program (MCSP), this is welcome news! We work every day around the world to ensure our interventions reach the most vulnerable populations, and understand the endorsement of misoprostol means more equitable access to and appropriate use of uteronics for countless women across the developing world.
Starting under USAID’s predecessor Maternal and Child Health Integrated Program and continuing under MCSP, we’ve employed innovative community based strategies to address PPH, such as advanced distribution of misoprostol for self-administration. And as our PPH prevention efforts have borne fruit, we have increasingly focused on improving access to and quality of treatment for PPH to combat maternal deaths. MCSP works closely with country counterparts to promote packages of high-impact PPH treatment interventions—including the uterine balloon tamponade—and strengthens the capacity of providers to manage PPH through simulation-based training.
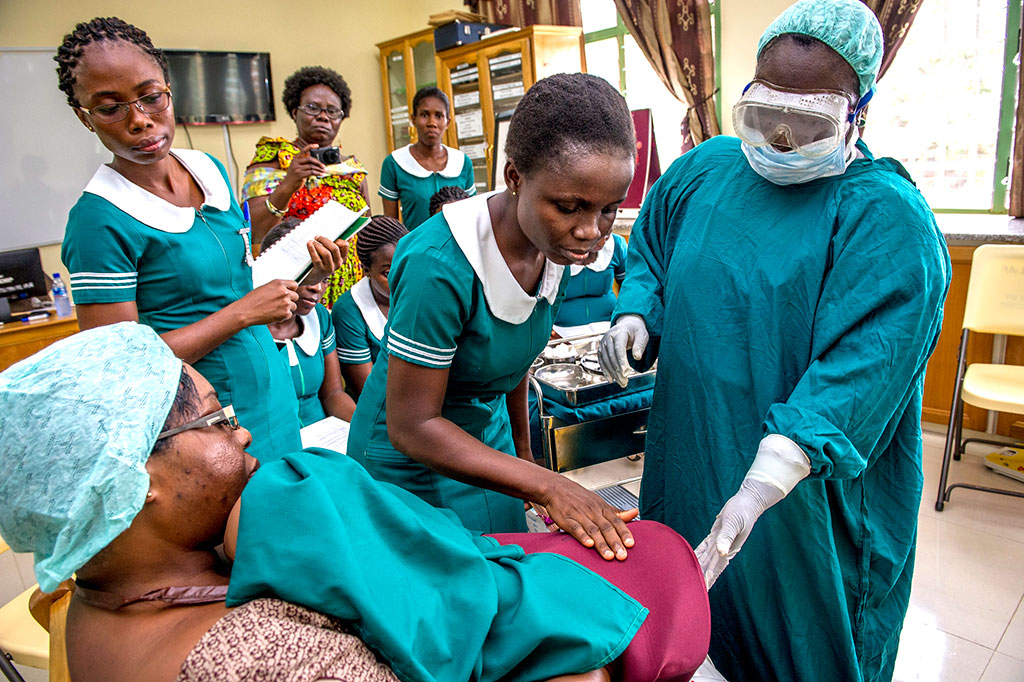
The WHO announcement opens an exciting new chapter in global health. While much work remains before every facility can guarantee a stable stock of viable oxytocin, the endorsement of misoprostol for the treatment of PPH will increase the availability of lifesaving care for some of the world’s most vulnerable women.
As part of our own comprehensive PPH strategy, MCSP continues to strengthen essential health system functions, with the goal of overcoming local system barriers to provision of high-quality care, effective referral systems, and trained providers. Please join the conversation on Facebook and Twitter to learn more about the great work we’re doing!

